How Do You Know if Your Antique Gold Jewelry is Really Gold?
Why is this guide different? While we will cover the basics of gold purity and gold testing, we will also share with you what else you should look for in order to determine whether your antique piece of jewelry is really gold.
First let’s start with some basics.
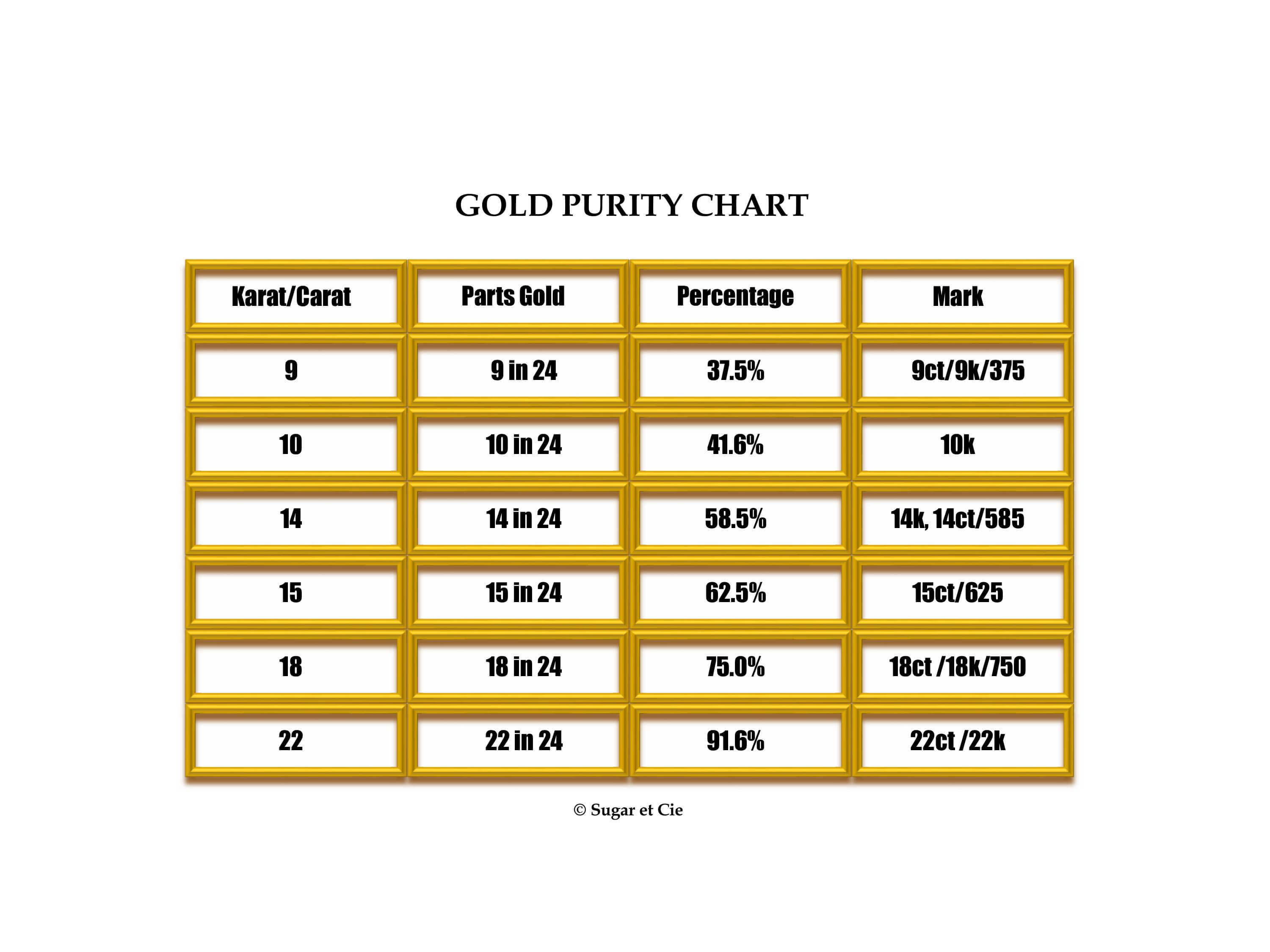
Gold Purity
Did you know that gold purity not only factors into the value of the piece, but also its color and its strength? Gold jewelry is not typically made in 24K (pure gold) due to its softness. It would not hold its shape.
When choosing the gold purity of your yellow-gold jewelry there are three competing factors to consider: strength, depth of color, and value. Gold of a lower carat has a greater percentage of alloy, and as a result is stronger than gold of a higher carat. Gold of a higher carat has a greater percentage of gold, and therefore a richer or deeper yellow-gold color.
Gold Color
Gold also gets its color from the alloys mixed with it: the type of alloys used and the percentage of each in the mix.
To give you an idea of which alloys are used in different colors of gold, we’ve created the chart below. Not every color or every carat is included, but it is a good illustrative guide.
Did you know there is such a thing as purple gold?
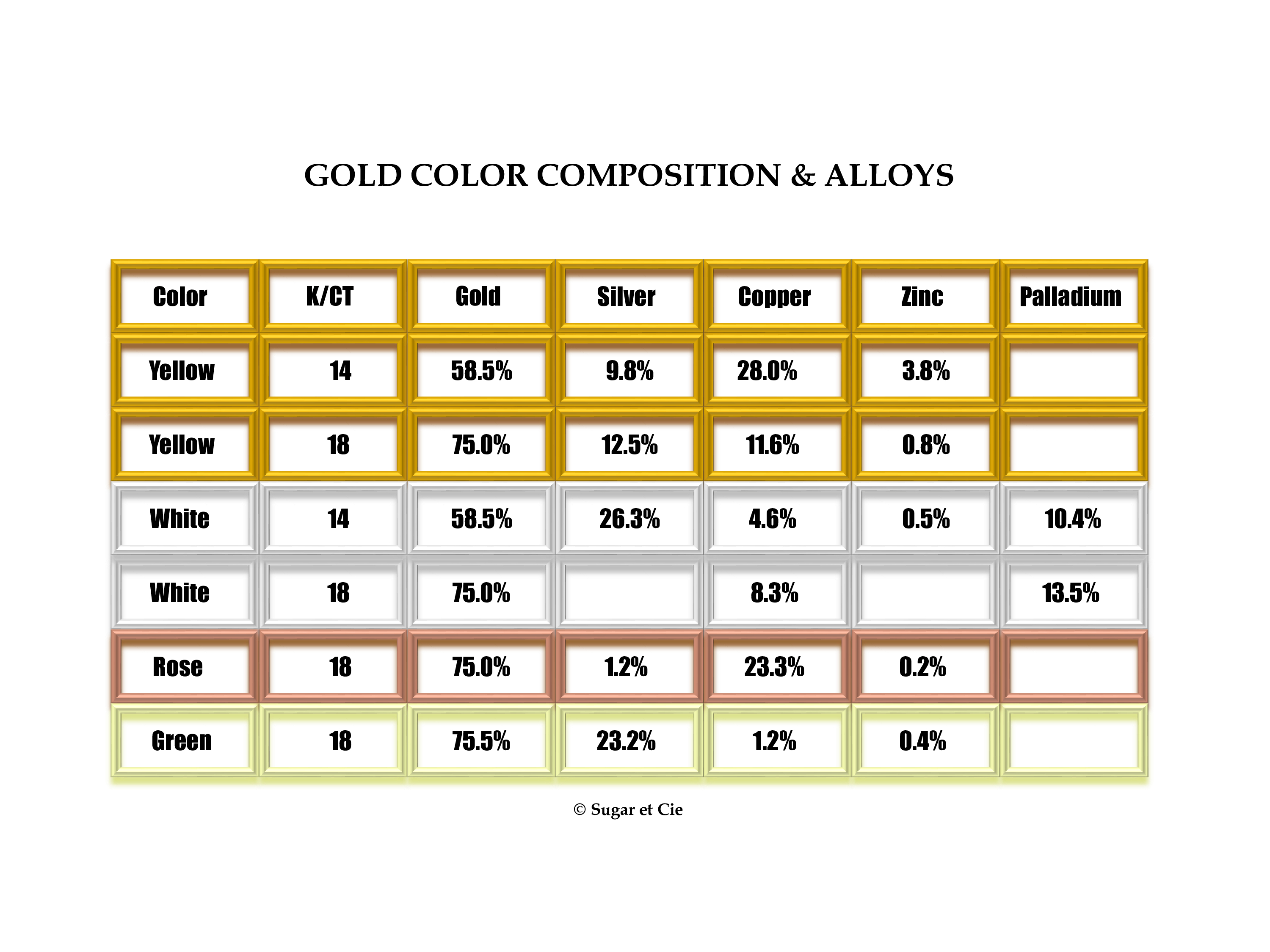
There are a large number of gold refineries in the U.S. that recycle and produce gold for resale. The percentages above are from Stuller. The percentages are supposed to add to 100%, but not all actually do. I asked Stuller about this and they said the percentages disclosed are slightly off due to the fact that the information is proprietary and they don't want others to copy their exact formula.
Despite this fact, most gold available these days follows a similar formula and the sources of alloys are mostly the same.
Why Does Modern Gold Look Different From Antique Gold?
In the 19th c., gold was alloyed by the individual jeweler. So the mix was custom and it explains why the hues found in antique gold jewelry often look different from modern gold.
Antique yellow-gold often looks “richer” or “warmer” in tone than modern yellow. And antique rose gold is often less pink than modern rose gold.
I asked Garfield Refining (see more about them below under Resources) what they thought might account for the color difference between modern and antique. They concurred, but added that the source and the quality of the alloy in the mix probably also had a big impact. The alloy was likely to have been of a higher quality or purer two centuries ago.
Patina is another factor. This is partially related to the alloy used and the oxidation that develops over time.
Antique Jewelry with Other Gold Finishes that are still Gold
Before we move on to testing, it’s worth mentioning that there are some interesting finishes and techniques that jewelers and artisans use(d) to give gold a different look or color. This can often be a clue to the period of the piece.
Silver or Platinum Topped Gold
Before white gold and platinum were introduced into the commercial jewelry industry, jewelers would use a thin layer of another metal over the gold to give the piece a white finish. During the Georgian or Victorian period, the metal used to accomplish this look was silver.
This Victorian diamond star brooch is such an example. The old mine cut diamonds are set in 15ct gold topped with silver. The silver layer gives the star a white appearance (with some oxidation), but you can see it is fabricated from gold if you look at the reverse side of the brooch/pendant.

© Copyright Sugar et Cie

© Copyright Sugar et Cie
During the Edwardian/Bell Époque Period a piece could still be topped with silver, but platinum was more frequently becoming the metal of choice. White gold was not commercially utilized in the jewelry industry until the Art Deco period (timing varied a bit from country to country). In the U.S., David Belais was credited with the patent of 18k white gold in 1918 (Jewelers' Circular March 10, 1926 Volume 92 p.69).
So if someone is claiming that a piece of jewelry is white gold and from the Victorian period, something isn’t quite right. It might be Victorian, but it is not white gold or it might be white gold, but it is not Victorian. Or perhaps it is none of the above.
Gold Wash
Gold wash refers to a thin layer of gold over some other metal. In some cases you might see a gold wash over silver or other base metal. And in some cases it might be gold, over gold. It is not always a “bad” thing. You just need to understand what you are looking at.
For example a solid gold antique chain might be mostly yellow-gold but you might see rose gold coming through especially at wear points on the chain. For some reason, the jeweler had a rose gold piece and decided to give it a yellow-gold wash. You can test it to confirm it. Just keep in mind that another color coming though, isn't always a bad thing. We’ll talk more about this later in Testing your Gold.
Bloomed Gold
Bloomed Gold refers to a piece of gold jewelry with a particular type of finish. The finish is achieved via a method of alloy depletion. Something with a bloomed gold finish usually has a rich yellow color with a velvety matte finish. Pictured below is a pair of 19th c. Etruscan Revival earrings in 18 K gold with a higher-carat bloomed gold finish.

© Copyright Sugar et Cie
The finish is achieved by dipping the piece in an acid formula. The solution eats away at the alloy and mattifies the surface by microscopically etching it. The process leaves behind a thin layer of pure gold. The result is the look of high-carat gold.
Oxidation
Here’s a myth. Gold doesn’t tarnish or oxidize so if there is oxidation, it is not gold. Well pure gold doesn’t, but anything less than 24k can discolor over time.

Courtesy of Auktionshaus Mars
It depends on the gold carat and the type of alloys used. The reason to mention this is that when you are inspecting a piece, if you see discoloration and you are able to polish it away, then it is likely just oxidation, rather than solder from a repair or base metal showing through.
The image above is of an antique brooch from the turn of the 18th c. It is fabricated from silver and 14K gold. The discoloration is an example of oxidation.
Note, be careful not to polish or clean a piece that has a finish that you don’t want to damage: Bloomed Gold or the blackened patina that often develops on silver topped gold (see the diamond star image). Be sure to specify this if you take an antique piece in to a jeweler for any modifications or cleaning.
The blackened patina is often considered desirable and modern artisans have been known to try to recreate the look through chemical treatments or rhodium plating.
Gold Look-alikes
The following are gold “look-alikes” that are not solid gold or not gold at all.
Plated: A piece of jewelry that is gold plated consists of a base metal with a thin layer of gold. There are several steps in the process but basically the piece of jewelry (made of some other metal) is dipped into the solution and the gold sticks to the base metal via hydrolysis. The plating thickness is a factor of time in the solution.
Rolled: Rolled gold jewelry is created by bonding (via heat) two thin sheets of gold with a base metal (e.g. silver or brass) sandwiched in between. While still thin, it is much thicker than the metal deposited via plating.
Pinchbeck: Pinchbeck gold is not gold at all, but it is important to be familiar with it if you are a collector of antique jewelry. It is comprised of the same alloys used to create brass (copper and zinc) yet it looks like gold and wears like gold. It was developed by Christopher Pinchbeck in the 18th c. He was notoriously secretive about his formula and manufacturing process.
So...Is it Gold? Gold Testing
Let’s move from Antique Gold Characteristics to Gold Testing.
The steps listed below assume that you do not wish to do anything destructive and the fall into the category: Non-Destructive Testing. There are other tests that can be done if your goal isn’t to perfectly preserve the piece.
Step 1: Inspect Your Jewelry
The easiest and best place to start is to look for a Gold Hallmark or Stamp. You’ll need to pull out your loupe for this. To learn more about Gold Hallmarks read our Guide: Interpreting Hallmarks on Jewelry, Part I.
The challenge is not everything is hallmarked or stamped. Even in England and France where gold hallmarking was more prevalent not everything has a purity mark.
Step 2: Other Signs
What do I look for next? Well I look for tell-tale signs. If it is antique and it hasn’t been locked away in a box, it should have wear. If there is wear, I look for base metal showing through at the wear points with my loupe. In the case of yellow or rose gold, I am looking for something white, silver, basically something not gold in color. As mentioned, you don’t necessarily need to be concerned if you see another gold color. Some rose gold will be washed with yellow and some gold may be oxidized in areas.
Step 3: Acid or Touchstone Testing
The most widely used, non-destructive form of testing is acid testing or touchstone testing. You can buy a kit. I have included links in the Resources Section below for kits with instructions that you can purchase from a reputable site.
It does take some experience and education, but it is something you can do in the comfort of your own home. And if you really want to be sure it is gold, you do have to get to a spot that is beneath the surface. I don't advise that you undertake this on your own if you care about causing damage to the piece of jewelry. I won’t go into great detail here about the details of how it works as there are plenty of great videos out there.
Use care to find an area on your piece that is appropriate (edges for example where the rubbing won’t be obvious) or an area of the piece you think is suspect. Next rub the selected area onto the stone. Decide upon the gold carat you would like to test for and select the matching sample gold needle provided in the kit.
Rub a similar line with the selected gold needle near the gold sample you just rubbed on to the stone. An acid solution labeled the same as the testing needle is dropped on to both gold rub marks made on the stone. Study the reaction. Does the gold mark hold or does the acid eat away at it? Most advise starting with the highest carat acid and working your way down.
If you would like to read more about Touchstone Testing, here is a more in-depth article on GIA's website: Bench Tip #9: Use the Touchstone Method for Testing Purity in Karat Gold.
Step 4: XRF Testing
If you are still concerned, the next level, non-destructive test is XRF. Not everything needs an XRF test. What is XRF you ask?
It stands for X-Ray Fluorescence. It is a non-destructive analysis that measures the fluorescence x-ray emitted from the sample during testing. You place the item in the machine, turn the machine on, a radiation beam is directed at the piece of jewelry, measurements are made and reported.
The results will tell you the composition of the metal: what percentage is gold, the types of alloys used, and the percentage of each. It is always fascinating to me to see what alloys and in what percentage were incorporated 100 plus years ago.
If you are outside the industry, it can be a little challenging to get an XRF analysis. It is an expensive machine (ten thousand plus) and most jewelers don’t have the need to own one. Someone who does a high volume of testing on a regular basis is more likely to have one (e.g. a pawnshop, an Assay Office in the U.K.).
Step 5: Get Access to an Expert
Free: Does your city have a Pawn Shop or Refinery with an XRF machine? Be honest about your intentions regarding whether or not you intend to sell or refine. If you are friendly and don’t constantly come in with pieces for testing, you might find that they are willing to test your gold for you for free or a small fee.
Free: Check to see if you have an Auction House in your area that offers "Valuation Days". The experience will vary quite a bit from Auction House to Auction House. You may get great information on your piece ranging from an opinion of value and willingness to test it for you, to a very big range of value with a lot of conditional statements. However, it is a free service with no commitment to consign required. Keep in mind it will be verbal and non-binding.
Paid: Get an appraisal. You might be surprised that the cost to have something appraised is not as much as you thought. Depending upon your local market and the complexity of the piece that you present, the fee for a single piece could be as low as $75 ranging up to $200 or possibly more. You will walk away with a written report that not only includes information on the gems and the metal in your piece, but also a statement of value. Be sure to be clear on the basis of value that you are interested in (e.g. Insurance/Replacement value, Fair Market Value, or Liquidation Value).
Resources:
FINE ANTIQUE, ESTATE & MODERN JEWELRY: Sugar et Cie
GOLD TESTING KITS:
Otto Frei Gold Test Kit With Pre-Mixed Acids (10k/14k/18k)
Otto Frei Gold Test Kit with Pre-Mixed Acids (10k/14k/18k/22k/Silver/Platinum), Scale
Precious Metal Refining: Garfield Refining (Philadelphia, PA)
Sources:
"White Gold Patent Case Decided in Court of Appeals" Jewelers' Circular March 10, 1926 Volume 92 p.69
Stuller: Metal Composition Chart
ThermoFisher Scientific: " What is XRF and How Does it Work?"
Sheffield Assay Office: www.assayoffice.co.uk

